* This is the latest installment of “Problematica,” and the first to actually discuss problematica. It is written by Max Dresow…
It began with a blob. No more than 12 mm long and 9 mm across. The whole thing looked a bit like flattened chiton: one of those humble mollusks clad in overlapping plates that you find sticking fast to seaside rocks. But it wasn’t a chiton. And according to a popular view of the fossil record, it wasn’t supposed to be there.
During the 1870s, the oldest fossil-bearing strata on the planet were known as “Primordial.” This was the name that had been given to the “first” living fauna by the great Bohemian paleontologist Joachim Barrande. There were Primordial strata in eastern Newfoundland, close to place where the blobs had been collected. But the blob-bearing rocks clearly dipped beneath the Primordial strata in the geological succession. This made the blobs perhaps the oldest items in the fossil record, assuming they were the remains of past life as opposed to sedimentary processes playing tricks.
GSC 221: a specimen of Aspidella terranovica likely collected by Alexander Murray in the 1860s
The discoverer of the fossils was a man named Alexander Murray. A survey geologist and first director of the Geological Survey of Newfoundland, Murray began collecting the disk-like structures around 1866 in the area of St. John’s (Minicucci 2017). He first described them in his “Report upon the Geological Survey of Newfoundland for the Year 1868.” There he observed that the fossils were “organisms of a low type” perhaps allied to the Cambrian taxon Oldhamia, a presumed bryozoan. However, Murray was not a paleontologist and declined to give more than a perfunctory description of the specimens. This task fell to Murray’s countryman, Elkanah Billings, a one-time barrister who had given up his legal career to become Canada’s first professional paleontologist (Clark 2004).
Alexander Murray (left) and his countryman Elkanah Billings (right)
Billings published the first formal description of Aspidella terranovica in his journal, The Canadian Naturalist. There he observed that specimens of Aspidella are “small [and] ovate… five or six lines in length and about one-fourth less in width. They have a narrow ring-like border, within which there is a concave space all round. In the middle there is a longitudinal roof-like ridge, from which radiate a number of grooves to the border. The general aspect is that of a small Chiton or Patella, flattened by pressure.” Yet it is unlikely, Billings thought, “that [Aspidella is] allied to either of these genera.” The structures were biological in origin—of this he was confident. As to what exactly they were, he was hesitant to speculate.
Billings’s drawing of Aspidella in the Canadian Naturalist (1872 volume vi, 478)
Curiously, Aspidella never became a major object of controversy despite its undoubted antiquity. Perhaps this was because of the hubbub generated by another Canadian fossil, Eozoön canadense, the “dawn animal of Canada” (depicted in the above title banner). Debates about Eozoön burned hot and bright after 1865, until the discovery of specimens in volcanic ejecta cinched the case for an inorganic origin in 1894. No similar drama surrounded Aspidella, although in the end the unassuming blob inspired almost as many divergent interpretations. Charles Doolittle Walcott reckoned it biological before coming to view it as a mere “spherulitic concretion [or mass of mineral cement].” Sir William Dawson, champion of Eozoön, speculated that it “may have been a mollusk… or some obscure form of crustacean,” although he too later cooled on a biological interpretation. George Frederic Matthew, father of William Diller, wrote to Alpheus Packard that “[Aspidella] seems to me a slickensided mud concretion striated by pressure.” By contrast, the feisty Jules Marcou declared it “the only [Precambrian] specimen of an organic structure certain and indisputable.”
Aspidella, then, was a Rorschach test for expert observers. For some it was an organic structure of uncertain affinity. For others it was an imposter. For still others it was an enigma that was impossible to penetrate.
Another specimen of Aspidella, this one containing two individuals (image credit: Marc Laflamme)
Aspidella is now believed to be an authentic fossil, although it remains unclear what exactly it was (Gehling et al. 2000). What is fairly certain is that it is a body fossil of some sort, perhaps a holdfast of a frond-like organism whose connecting stem is usually missing. Since it’s old and circular you can bet that it’s been compared to a jellyfish, but this interpretation is hardly persuasive anymore. More plausible is that it represents a ring-shaped microbial colony, although “the geometric regularity of individual specimens… differ[s] significantly from those observed for interfering bacterial or microbial mat colonies with concentric growth” (Gehling et al. 2000, 444). Thus, one hundred and fifty years after Billings described Aspidella, the question of its taxonomic status remains unresolved. This is a problem that is common among fossils that date to the last major interval of the Neoproterozoic: the Ediacaran Period (635–538.8 Mya).
Ediacaran fossils and the problem(s) with problematica
Assuming Aspidella is an authentic fossil, it has the distinction of being the first fossil from the Ediacaran Period to have received a name and description. There have since been many others. Ediacaran fossils have been found on every continent besides Antarctica. The most ancient are in excess of 570 million years old, dating from just after the Gaskiers glaciation. They are prevalent in rocks spanning the next 30 million years or so, right up to the base of the Cambrian Period (538.8 Mya). Then they mostly disappeared. What remains is a collection of impressions—mostly disks, fronds, and blobs—that have puzzled paleontologists for the better part of a century. Were they cnidarians, segmented worms, primitive arthropods, fungi, or even lichens? Or were they maybe microbial colonies, protists, or unique designs representing a failed experiment in the evolution of large size? All these interpretations and more have been mooted by paleontologists, recapitulating on a broad scale the ambiguity surrounding Aspidella.
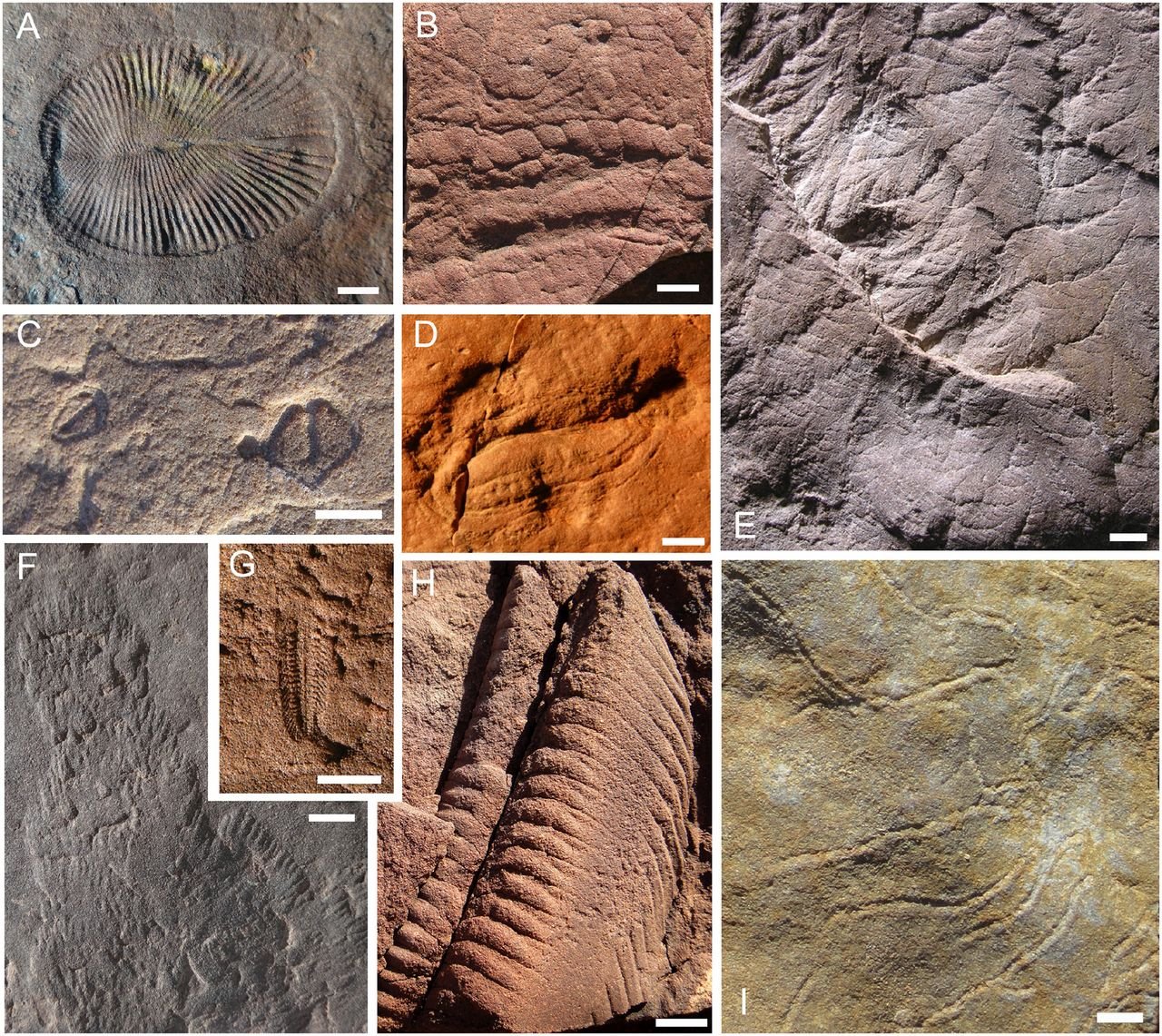
Characteristic Ediacaran fossils from the Flinders Range, South Australia. (A) Dickinsonia costata, (B) Funisia dorothea body casts and external molds, (C) Parvancorina minchami, (D) Kimberella quadrata, (E) Multilayered sandstone case of Bradgatia sp, (F) scratch traces produced by Kimberella, (G) Spriggina floundersi, (H) Internal cast of Pteridinium simplex, and (I) an ichnofossil, Helminthoidichnites isp. (Image credit: Droser and Gehling 2015, Fig. 1)
The technical term for a fossil like Aspidella is a “problematic fossil” or “problematicum.” This refers to a fossil that defies robust taxonomic placement, and is likely to receive the designation incertae sedis (“of uncertain placement”) at whatever level of classification the uncertainty resides. I am especially fond of the term “problematica,” as you will have guessed—it is just so wonderfully self-explanatory. Yet as Stefan Bengtson writes in a perceptive essay, there is not one problem associated with problematic fossils but several. Problematic fossils resist taxonomic placement for at least three reasons:
[It] may be the fault of the investigator, in which case the Problem is trivial and does not deserve [a] capital P. It may be the fault of the fossil, in that it does not reveal enough of its character to the investigator. [Or] it may be the fault of our taxonomic concepts, in which case it starts to become interesting. (Bengtson 1986, 3)
To this I would add a fourth category. Sometimes, problematic fossils resist placement for reasons having less to do with the details of preservation (“the fault of the fossil”) or the contours of taxonomic concepts, as with evolution itself. If a taxon resides on the stem of a living clade, it may be the case that the traits used to define the basal node of that clade had yet to evolve. (And to add to the confusion, some traits not shared by the living members of the clade might be present.) The problem is mitigated by the concept of a “stem group,” which helps to make sense of taxa bearing a confusing medley of characters (see the figure, below). But it strikes me that refined taxonomic concepts will not suffice to de-problematize all problematica, especially when the taxa in question reside deep in evolutionary trees. Here it is evolution that is the problem, not taxonomy— or at least not taxonomy alone.
When it comes to Ediacaran fossils, all of the major problems with problematica are in play. To begin, most Ediacaran fossils are preserved as casts and molds in relatively coarse sediment, and this has led to a considerable loss of anatomic detail. An especial problem is the absence of readily identifiable anatomical features, which is partly a function of information loss and partly a matter of evolution. Ediacaran fossils are ancient, nestled deep in the evolutionary tree of animals (assuming they are indeed animals). This means they did not possess many of the features characteristic of living groups, including those traits that define the basal nodes of surviving clades. But then how should they be classified? Extinct basal taxa invariably fall outside classifications established on the basis of living forms: this is a straightforward feature of the geometry of evolutionary trees. Yet insofar as extinct basal taxa share at least some features with living members of a clade, it may be possible to locate these taxa on the stem of that clade. This requires, first, that enough features are preserved to conduct a phylogenetic analysis (the fossil must cooperate), and second, that researchers are able to identify relevant features as homologs of traits in living clades.
In phylogenetic systematics, the “stem [group]” refers to all the members of a total clade falling outside the “crown [group]”: the group of species comprising the last common ancestor of the living members of a clade and its decedents, living and extinct
The second point comes down to fossil interpretation. Ultimately, “fossils can only be interpreted in light of our knowledge of living species” (Jenner and Littlewood 2008, 1506). This is not to say that it is never useful to compare one fossil taxon to another—often it is. It is simply to observe that “the chain of comparisons must link to an extant organism [if it is to be informative] with regard to homology” (Donoghue and Purnell 2009, 180). And in the case of problematica, this causes serious difficulties. As Donoghue and Purnell observe: “the identification of anatomical homologies is contingent upon the systematic framework in which they are considered… [and] requires, among other criteria, an a priori hypothesis of grouping at some level before the process of comparative anatomical interpretation can proceed” (Donoghue and Purnell 2009, 180). The hypothesis does not have to be very specific—often it will be enough to know that the organism was a lophotrochozoan or a bilaterian as opposed to a mollusk or an arthropod. Still, to confidently pin down character identities, some interpretive (or “comparative”) model needs to be in place. The problem is that when a phylogenetic signal is weak (because of unfavorable preservation or great phylogenetic depth), the choice of an interpretive model is likely to be underdetermined. And this is a recipe for disagreement based on different modeling choices and their propagating consequences for comparative anatomical analysis.
The history of Ediacaran fossil interpretation is accordingly one of disagreement. As Budd and Jensen observed in 2017, “Probably no other fossils have generated such a range of diverse interpretations as the enigmatic ‘Ediacaran’ macrofossils… with possibly the only broad agreement being the absence of biomineralized hard parts” (Budd and Jensen 2017, 452, emphasis added). Bruce Runnegar agrees, writing in a recent review that “[for] almost 150 years, megascopic structures in siliciclastic sequences of terminal Precambrian age have been frustratingly difficult to characterize and classify” (Runnegar 2021, 1). In a later essay, I will consider how these problems of characterization and classification have been overcome, to some extent, in recent years. First, though, it will be instructive to look at the history of Ediacaran paleobiology in a bit more detail.
A Selective history of Ediacaran fossil interpretation
While fossils now dated to the Ediacaran Period were known from the 1860s, it wasn’t until the middle of the twentieth century that Ediacaran paleobiology began to take off. This was when Reginald (“Reg”) Sprigg found the first specimens of Ediacaria flindersi, among other fossils, in the Ediacaran Hills of South Australia. Sprigg was in the Ediacaran Hills on business; he was a survey geologist sent to map the area in search of valuable mineral deposits. But Sprigg was no stratigraphic grunt—he was also an amateur paleontologist with a keen interest in the early evolution of animals. So, when he discovered in some flaggy sandstones “queer markings suggestive of jellyfish” (as he wrote in his field notebook), he eagerly snatched them up (Sprigg 1988).
Sprigg’s “jellyfish,” Ediacaria flindersi (photograph by Sprigg, from Sprigg 1988)
Probably it was Sprigg’s exposure to dehydrating jellyfish on the beaches of Adelaide that disposed him to interpret his find as a cnidarian (Runnegar 2021). That and a geologic error: he believed the convex disk-like fossils to be preserved on bed bases (as impressions) rather than on bed tops, consistent with the jellyfish interpretation. Anyway, the jellyfish interpretation had legs, notwithstanding that Sprigg failed to drum up much enthusiasm for his fossils. One reason is that Sprigg assigned his fossils to the earliest (or “Eo”-) Cambrian, then believed to be an “age of jellyfishes.” This had the effect of rendering Sprigg’s fossils expected, unremarkable—just the kind of thing you were liable to find at the base of the Cambrian before the more advanced trilobites scuttled onto the scene. In any event, jellyfish they were to remain until the 1970s, when several linked developments suggested a different interpretation. (For more on the fate of the jellyfish interpretation, see the Coda.)
The Czech paleoartist Zdenek Burian’s painting of the Cambrian Sea (1960). Notice the jellyfish bobbing conspicuously in the foreground
The fossils that would later be known as “Ediacaran” began to receive more attention in the 1950s, when a schoolboy brought a leaf-like impression to the notice of an English geologist. (Actually, a schoolgirl seems to have discovered the fossil first, but she was ignored.) Around the same time, a world-class paleontologist, Martin Glaessner, began a sweeping program of research on Sprigg’s fossils in Adelaide. Glaessner was a transplant, an Austrian forced to leave Vienna in 1938 following the Anschluss. He seems not to have taken a serious interest in Sprigg’s fossils until the mid-1950s, at which point Sprigg had left the geological survey to set up a private consulting firm. In any event, it was Glaessner, not Sprigg, who brought the fossils to international renown: a development that had more than a little to do with a statigraphic revision that placed them in the Late Precambrian, previously thought to be almost devoid of complex macrofossils (Glaessner 1958).
Over the next two decades, Glaessner and his associate Mary Wade assigned most Ediacaran fossils to living groups on the strength of supposed structural analogies. Pancake-like Dickinsonia he described as a segmented worm (Glaessner 1961). Foliate Rangea he described as a “sea pen” (Glaessner 1959). Slug-like Kimberella (neé Kimberia) he assigned to the medusoids (Glaessner and Wade 1966). And the unusual Praecambridium he described as an arthropod (Glaessner 1961). Glaessner was under no illusion that these organisms were modern animals in drag—his decision to assign them to living groups was entirely a pragmatic one (Glaessner 1984). Still, it was so successful that he could claim near the end of his life that while “questions have been raised and doubts [expressed] about the placing of some species… no major changes in the assessment and composition of the fauna have been made in the last 25 years” (Glaessner 1984, 51).
Glaessner’s photograph of Rangea from Glaessner (1961), juxtaposed with a living “sea pen” (a soft coral)
That all changed during the 1980s when Adolf Seilacher entered the fray. Invariably described by his colleagues as “a marvelous observer” (Fortey 1997, 81) or even as “the finest paleontological observer now active” (Gould 1989, 312), Seilacher was not a specialist in Precambrian paleontology. Still, he had the gall to propose that the specialists had it all wrong—not just the details of particular analyses, but the most basic features of their interpretation.*
[* Possibly this is related to the other thing colleagues said about Seilacher: that he possessed a strong didactic streak “which comes from a conviction that he is right about every issue” (Fortey 1997, 81).]
Seilacher’s 1970 visualization of constructional morphology, integrating the historical (historisch-phylogenetischer), functional (ökologisch-adaptiver), and fabrication (bautechnischer) aspects of form
Seilacher’s interpretation of the Ediacaran biota was based on what he termed “constructional morphology” (Seilacher 1989). Regarded as a method, it involves the analysis of form in relation to three factors: history (“phylogenetic tradition”), adaptation (“biological function”), and Bautechnik (“morphogenetic fabrication”). The details of the analysis could be somewhat opaque, yet its strategy was to reconstruct the morphological design of extinct taxa without the help of metazoan analogies, and then to interpret this design in functional terms, again, without the help of analogies.* Using this strategy, Seilacher concluded that most of the iconic Ediacaran organisms, from the “worm” Dickinsonia to the “sea pen” Rangea, were representatives of an “exotic principle of organismic construction” unique to the Precambrian (Seilacher 1989, 230). This principle was based on a shared constructional element called a “pneu structure”: basically, a cylindrical tube filled with gelatinous fluid. Ediacaran organisms were compounded of many pneu structures quilted together to form “biological carpets” reminiscent of air mattresses. How they lived is a mystery. They seem to have lacked mouths and anuses, as well as sensory and locomotive organs. Perhaps they fed upon the microbial mats that lined the Precambrian seafloors, but Seilacher’s hunch was that they harbored microbes capable of extracting energy from seawater. In any event it is at this juncture that “the disadvantage of [a purely constructional] approach” was most evident, since all the investigator had to go on were the “basic principles of physiology,” and these provided less guidance for analysis than a proper interpretive model.
[* This reliance on metazoan analogies is where Glaessner went wrong (Seilacher thought). For example, whereas Glaessner interpreted some Ediacarans as sea pens, living sea pens have polyp-lined branches that permit water and suspended food particles to flow between them. In Ediacaran “rangeomorphs,” by contrast, the “polyps” seem to have been joined together in quilt-like structures that would have precluded this mode of feeding (Seilacher 1984).]
Seilacher’s hand-drawn illustration of “vendozoan” morphology, based on the shared constructional element of the pneu structure (middle row, center). According to Seilacher, vendozoans had three modes of growth—unipolar, bipolar, and radial—which enabled them to achieve a range of distinctive morphologies. Notice that this illustration depicts several of the iconic Ediacaran forms, including Dickinsonia (which Glaessner interpreted as a worm) and Rangea (formerly a sea pen).
Seilacher’s best known paper on the “vendobiont hypothesis” abstains from speculating about the taxonomic affinities of his carpet organisms. However, in his (1992) he makes the bold suggestion that they constitute a new kingdom of life (the Vendobionta) that became extinct at the end of the Precambrian. Possibly this is because the lifestyle permitted by their “quilted hydrostatic construction” became obsolete with the advent of mobile, bilateral predators. Such predators would have found the biological carpets easy marks, and as Seilacher infers, “even small bites would probably have led to fatal leakage from the living bags” (Seilacher 1992, 607). Unfortunately for the vendobionts, their capacity to mount an evolutionary response was constrained by another feature of their construction. This is that they became large by “[s]ubdividing a syncytial protoplasm by quilting.” Vendobionts were accordingly unicellular, and this served to limit the possibilities for tissue differentiation and specialization. Simply put, vendobionts could not hack it in a world of eyes, muscles, and jaws, and for this reason succumbed to mass extinction around the time of the Cambrian radiation (Seilacher 1984).
Seilacher’s interpretation was eventually discredited after a warm reception. But its impact on the field was considerable. Perhaps the most important thing it did was “turn the discussion of Ediacaran affinities from a monolith [into] a free-for all” (Narbonne 2005, 431). In the decades following Seilacher (1989), members of the “Ediacaran biota” were interpreted as protists (Zhuravlev 1993), fungi (Peterson et al. 2003), lichens (Retallak 1994), colonial prokaryotes (Grazhdankin 2007), and extinct photosynthetic “metacellulars” (McMenamin 1998). This represents a much wider range of interpretive models than Glaessner and company ever thought it prudent to entertain. Of course, not all of the interpretations were equally plausible, and throughout the interval most workers continued to regard the Ediacarans as animals, either the ancestors of living forms or members of an extinct group. Still, the interpretive diversity is striking, and points to the difficulties involved in justifying the choice of any particular model as the preferred one.
Some of the anatomical and morphological features of lichens and fungi, meant to provide an interpretive key to the body forms of (at least certain) “Vendobionta.” (From Retallack 1994)
It may be instructive, in concluding this section, to examine one of the phylogenetic interpretations that emerged in the wake of Seilacher’s intervention. In 2003, the paleontologists Kevin Peterson, Ben Waggoner, and James Hagadorn proposed that “some of the more locally abundant and geographically widespread [Ediacaran] taxa, including Charnia, Charniodiscus, and Aspidella, [are] best understood using modern fungi for comparison” (Peterson et al. 2003, 127). They motivated the claim by stating that “most, if not all, of the Ediacaran organisms from the Avalon Peninsula of Newfoundland cannot be understood using metazoans as analogs.” The reason was that Mistaken Point organisms possessed a strange collection of characteristics. They were evidently “[incapable] of movement; they either grew embedded within sediments or attached themselves to soft substrates [without burrowing] into them; and they may have possessed cell walls” (130). They also lived well beneath the photic zone, meaning that however they got their energy, it wasn’t through photosynthesis. “The only major clade of multicellular organisms that fits all of these criteria, and is not photoautotrophic, is the Fungi.” So, “[a] fungal model appears to be the most applicable model for some of the Mistaken Point biota, at least from a structural and physiological standpoint.”
Representatives of two Ediacaran taxa: on the left, Aspidella from Newfoundland, and on the right, Cyclomedusa from South Australia. In Peterson et al.’s analysis, the thing to notice is the sand accumulations in the middle of the specimens (marked with asterisks on the Aspidella specimens), which they interpret as analogous to the aged zone of a modern fungal mycelium: “Sediment is being deposited here because of the absence of living tissue” (Peterson et al. 2003, 132)
Does this suggest that organisms like Aspidella were literally fungi? Not necessarily. Perhaps they were only fungus-like, in the way that certain “slime molds” resemble fungi while laying outside the total group Fungi (Peterson et al. 2003). Or maybe they were stem fungi—a conclusion, the authors observe, which is roughly consistent with molecular clock estimates that place the origin of the fungi before 700 Mya. In any event, “the recognition that other living systems might provide a better model to understand the biology of some Ediacaran taxa will eventually shed insight into their proper phylogenetic position(s)” (134). When a problem has resisted solution for as long as this one has, it is reasonable to search for new interpretive models.
the last ten years
This little survey provided only thumbnails of a richer history. It neglected to mention Hans Pflug, for example, who assigned a number of frondose taxa from Namibia to a new phylum, “Petalonamae,” thought to reside between animals and plants on the evolutionary tree (Pflug 1973). Then there is Mikhail Fedonkin’s suggestion that segmented animals (“Articulata”) evolved from Ediacaran ancestors with an offset form of bilateral symmetry, representing the extinct phylum “Proarticulata” (Fedonkin 1985). (These in turn were held to have evolved from radial forms, representing the dominant animal body plan at the time.) More recently, Martin Brasier and Jonathan Antcliffe have argued that many “Glaessnerian species” are “not species at all… but mere organs, different growth stages… of a single taxonomic unit” (Brasier and Antcliffe 2004, 1115). Tellingly, Brasier and Antcliffe motivate their proposal by pointing to “the dangers of the popular ‘analog’ approach,” much as Seilacher and Peterson et al. did. The question is whether this is just frustration talking or whether the standard logic of fossil interpretation really does break down in the strange waters of the Precambrian.
Two phylogenies from Dunn and Liu (2019). The one on the left depicts Fedonkin’s Proarticulata hypothesis, with an “X” indicating clades that are now defunct (in this case, both Proarticulata and Articulata, and the clade comprising both taxa). The one on the right depicts Pflug’s Petalonamae hypothesis, with two taxa of “Petalo-organisms” separating animals and plants (note: animals and plants are no longer regarded as extant sister taxa)
What is certain is that 150 years after Billings described Aspidella, the Ediacaran enigma continues to exercise paleontologists. Much has been learned in recent decades, both about the Ediacarans themselves and about the world they inhabited. We now have a much better understanding of Ediacaran anatomy and diversity; about environmental conditions in the Late Neoproterozoic; about the mechanics of soft-bodied fossil preservation; and about the likely divergence times of major clades. Still, most Ediacaran taxa remain phylogenetically unresolved, and this frustrates attempts to pump them for information. If their affinities could be settled, light would be thrown on two major areas of interdisciplinary research: the origin of animal body plans and the evolution of novel traits. This is why Dunn and Liu (2017) call the problem of Ediacaran phylogeny “one of the biggest unresolved challenges in palaeobiology.”
But now I have to come clean. In discussing the affinities of the Ediacaran macrofossils, I have buried the lede. I haven’t actively misled you. The Ediacaran engima is every bit as sticky as I’ve made it out to be, for just the reasons I’ve given. However, in the past decade, the idea that most Ediacaran fossils are the remains of primitive animals has gained in plausibility, and now represents a broad consensus in the community. How can this be given everything I’ve just said? That is the subject I plan to address in a later essay, for which a link will be placed here when it is available.
CODA: Aspidella, Ediacaria, and the Fate of the Jellyfish Interpretation
When Elkanah Billings described the first specimens of Aspidella, he observed that “[their] general aspect is that of a small Chiton or Patella,” but warned that it is unlikely that Aspidella is allied to either of these genera. His hunch seems to have been confirmed by later researchers. No one today thinks that Aspidella is a mollusk, and indeed no one has taken the suggestion seriously for a long time. Instead, were you to ask someone during the last century what Aspidella was, you would have received one of two answers. The first and more common would have been that Aspidella is an artifact—perhaps the result of gas bubbling out of sediment, or else a suction mark or concretion. This was Martin Glaessner’s answer, as well as that of the eminent paleontologist and Precambrian fossil debunker Preston Cloud. The other would have been that Aspidella is a jellyfish, a medusoid impression comparable to some specimens described by Glaessner and Mary Wade. Few investigators seemed all that happy with this notion. But anyway it was an option, and one that was broadly congruent with what was then known (or believed) about Ediacaran paleobiology.*
[* Recall that in the traditional interpretation of the Ediacaran fauna, most taxa could be assigned to three groups: the jellyfish, the soft corals, and the annelids. Foliate taxa like Rangea were assigned to the soft corals. Segmented forms like Dickinsonia were presumed to be annelids. And pancakes and blobs were regarded as medusoids. The latter included Ediacaria, Medusina and Spriggia (not to be confused with the supposed-annelid Spriggina), as well as Cyclomedusa and Kimberella.]
The notion of Ediacaran jellyfish was first mooted by Reg Sprigg, who seems to have entertained no other interpretation of the fossils later called Ediacaria. It was shared by two private collectors, Ben Flounders and Hans Mincham, who in Glaessner’s telling “brought to light not only large numbers of presumed jellyfish but also segmented worms, worm trails and the impressions of two different animals that bear no resemblance to any known organism” (Glaessner 1961, 73). Flounders and Mincham’s discoveries are what got Glaessner into the game, and what resulted in a Precambrian date being placed on the Ediacaran fauna. What followed was a burst of research that saw Glaessner lay down the outlines of an interpretation that would guide Ediacaran paleobiology for the next 25 years. He summarized his early views in a Scientific American article of 1961, in which he declared that the Ediacaran fauna contains “jellyfish representing at least six and probably more extinct genera.” This amply “justifies the characterization of the Pre-Cambrian as the ‘age of the jellyfish.’” Nonetheless, he cautioned that the term “jellyfish” applies to a number of loosely related forms belonging to the coelenterate phylum, and that “none of the Pre-Cambrian medusae can be tied with any confidence to living order, suborders, or families.” Ediacaran jellyfish were incertae sedis below the level of classes, or perhaps phyla.
An image from Runnegar (2021), which juxtaposes Reg Sprigg’s drawing of Ediacaria (top left) with an illustration from Glaessner’s Scientific American article of 1961. Notice the medusoids swimming in the foreground while others are stranded on the beach
The jellyfish interpretation was remarkably stable over the next quarter century or so. Seilacher, as usual, shook things up. His argument against Edicaran medusoids had both a negative and a positive component. On the negative side, Seilacher observed that living jellyfish move by contracting a ring of muscles at the margin of their bodies, permitting a kind of gliding locomotion. Inside these muscles lie radial sutures that transport food to a ventrally located mouth. But this sensible setup is reversed in Ediacaran “jellyfish,” with the radial elements (“sutures”) lying outside the concentric ones (“muscles”). This makes it difficult to understand how the animal could have moved or eaten, which is to say, difficult to understand how it could have existed at all.
Seilacher did not deny that there were coelenterates among the Ediacaran biota. Yet he doubted that the impressions interpreted as medusoids were in fact medusoids. Some of the fossils, he observed, were preserved in epirelief—as bumps rather than depressions—and these he interpreted as the “sandy skeletons” of primitive sea anemones. (For Seilacher, this represented a novel kind of architecture—a “rock-in-a-sock”—unique to the extinct class of animals, “Psammocorallia.”) Then there were radial vendozoans like Cyclomedusa, which sprawled on the seafloor, flat as baking sheets. No jellyfish present, then: just biological carpets and rocks-in-socks. This was the positive argument.
Another of Seilacher’s hand-drawn illustrations, this one featuring “rocks-in-socks” (upper left) along with a variety of supposed vendozoans
The negative argument was generally convincing; the positive one was not (Gehling 1990). Still, the jellyfish hypothesis hung on until about the turn of the century, when two pieces of work dealt it a heavy blow (Runnegar 2021). The first of these was a reinterpretation of the depositional environment of the Ediacara Member of the Rawnsley Quartzite, home of the iconic soft-bodied fossils of South Australia. Using a new sequence stratigraphic framework, Gehling was able to show that “[most] fossils are impressions on the soles of storm sandstone beds, deposited on delta slopes between fairweather and storm-wavebase.” It just isn’t the case that impressions of Ediacaria and other “medusoids” were left by jellyfish stranded by tides, as Sprigg had supposed.
The other piece of work was a collaboration between James Gehling and the Canadian paleontologists Guy Narbonne and Michael Anderson. This was the study that established Aspidella as an authentic fossil, and (probably) a body fossil. It was prompted by a renewed interest in the Ediacaran fossils of Newfoundland, like those adorning the rugged cliffs of Mistaken Point. Many of these were unmistakably biological, preserved in situ and in exquisite detail. They were also old: between 580 and 560 My, within the temporal range of Aspidella. This meant that Aspidella could not be dismissed as an inorganic structure strictly on the basis of its age. But there was more. Careful mapping of the Neoproterozoic strata of Newfoundland revealed Aspidella to have a restricted stratigraphic distribution. (It was not present continuously throughout the column, even after correcting for the availability of suitable lithofacies.) Two hypotheses thus needed to be distinguished: either Aspidella was “the impression of a soft bodied organism, or it was a non-actualistic sedimentary structure that ceased to be preserved after the advent of Cambrian ecosystems.”
To distinguish these hypotheses, Gehling and his collaborators “stud[ied] stratal and bedding surface distributions, variation in shape and cross section, [and] co-occurrence of other body fossils.” They also made comparisons “with Ediacaran fossils from the more diverse assemblages as well as with known discoidal forms of mechanical origin.” What they concluded was that Aspidella is best interpreted as a body fossil: a conclusion they presented as basically confirmed. They also found that Aspidella exists as a variety of preservational morphs including flat disks, negative hyporeliefs, and epireliefs. These morphs grade into one another, suggesting that the form represented by the “type morph” of Aspidella “[is] an end member of a plexus of morphs for which a single biological model is sufficient.” Their preferred interpretation is that these these morphs represent the holdfasts of frond-like organisms. Anyway, Aspidella is not a jellyfish.
Different preservational morphs of Aspidella, their features determined by the body size of the living organism along with prevailing sedimentary conditions
There is one last thing to say. Several of the preservational morphs of Aspidella have names that should be familiar. These include Ediacaria (Sprigg’s original “jellyfish”) and Spriggia, which Glaessner interpreted as a medusoid. As Gehling et al. write, “Once the Aspidella morphs have been retrodeformed, they cannot be distinguished from other Ediacaran discoidal taxa, and would be identified variously as Ediacaria, Cyclomedusa, Spriggia, Tirasiana, Irridinitus, or Planomedusites if found in other Ediacaran sites around the globe” (442). The conclusion, again, is that these names pick out different regions of a morphospace in which a range of structures are produced by specimens differing in body size and residing in different sedimentary environments.
This is how the “Age of Jellyfish” ends. Not with a bang but a whimper.
References
Bengtson, S. 1986 The problem of the Problematica. In A. Hoffman and M.H. Nitecki (eds.), Problematic Fossil Taxa. Oxford: Oxford University Press, 3–11.
Brasier, M. and Antcliffe, J. 2004. Paleobiology: decoding the Ediacaran enigma. Science 305:1115–1117.
Budd, G.E. and Jensen, S. 2017. The origin of animals and a “Savannah hypothesis” for early bilaterian evolution. Biological Reviews of the Cambridge Philosophical Society 92:446–473.
Clark, T.H. 2004. Elkanah Billings (1820–1876)—Canada’s first paleontologist. In R.W. Macqueen (ed.), Proud Heritage: People and Progress in Early Canadian Geoscience. Geological Association of Canada, Reprint Series #8, 47–49.
Donoghue, P.C.J. and Purnell, M.A. 2009. Distinguishing light from heat in debate over controversial fossils. Bioessays 31:178–189.
Droser, M.L. and Gehling, J.G. 2015. The advent of animals: the view from the Ediacaran. PNAS 112: 4865–4870.
Dunn, F.S. and Liu, A.G. 2017. Fossil focus: the Ediacara Biota. Paleontology [Online] 7. https://www.palaeontologyonline.com/articles/2017/fossil-focus-ediacaran-biota/
Dunn, F.S. and Liu, A.G. 2019. Viewing the Ediacaran biota as a failed experiment is unhelpful. Nature Ecology & Evolution 3:512–4.
Fedonkin, M.A. 1985. Precambrian metazoans: the problems of preservation, systematics and evolution. Proceedings of the Royal Society, Part B 311:27–45.
Fortey, R.A. 1997. Life: An Unauthorized Biography. London: HarperCollins Publishing.
Gehling, J.G. 1990. The case for Ediacaran fossil roots to the metazoan tree. In B.P. Radhakrishna (ed.), The World of Martin Glaessner, 181–224. Bangalore: Geological Society of India.
Gehling, J.G., Narbonne, G.M., Anderson, M.M. 2003. The first named Ediacaran body fossil, Aspidella terranovica. Palaeontology 43:427–456.
Glaessner, M.F. 1959. Precambrian Coelenterata from Australia, Africa and England. Nature 183:1472–3.
Glaessner, M.F. 1961. Pre-Cambrian animals. Scientific American 204:72–8.
Glaessner, M.F. 1984. The Dawn of Animal Life. A Biohistorical Study. Cambridge: Cambridge University Press.
Glaessner, M.F. and Wade, M. 1966. The late Precambrian fossils from Ediacara, South Australia. Palaeontology 9:97–103.
Grazhdankin, D. 2007. Ediacaran microbial colonies. Lethaia 40:201–210.
Gould, S.J. 1989. Wonderful Life: The Burgess Shale and the Meaning of History. New York: W.W. Norton & Co.
Jenner, R.A. and Littlefield, D.T.J. 2008. Problematica old and new. Philosophical Transactions of the Royal Society, Part B 363:1503–1512.
McMenamin, M.A. 1998. The Garden of Ediacara: Discovering the First Complex Life. New York: Columbia University Press.
Minicucci, J.M. 2017. Elkanah Billings: The lawyer who revealed the ancient life of the past. Geosciences Canada 43. https://id.erudit.org/iderudit/1037741ar
Narbonne, G.M. 2005. The Ediacara biota: Neoproterozoic origin of animals and their ecosystems. Annual Review of Earth and Planetary Science 33:421–42.
Peterson, K.J., Waggonner, B., Hagadorn, J.W. 2003. A fungal analog for Newfoundland Ediacaran fossils? Integrative and Comparative Biology 43:127–136.
Pflug, H. 1973. Zur Fauna der Nama-Schichten in Südwest-Afrika. IV. Mikroskopische Anatomie der Petalo-Organismen. Palaeontographica Abteilung A 144:166–202.
Retallack, G.J. 1994. Were the Ediacaran fossils lichens? Paleobiology 20:523–544.
Runnegar, B. 2021. Following the logic behind biological interpretations of the Ediacaran biota. Geological Magazine. doi.org/10.1017/S0016756821000443.
Seilacher, A. 1984. Late Precambrian and Early Cambrian Metazoa; preservational or real extinctions? In H.D. Holland and A.F. Trendall (eds.), Patterns of Change, 159–68. Berlin. Fed. Republic Ger.
Seilacher, A. 1989. Vendozoa: organismic construction in the Proterozoic biosphere. Lethaia 22:229–239.
Seilacher, A. 1992. Vendobionta and Psammocorallia: lost constructions of Precambrian evolution. Journal of the Geological Society, London 149:607–13.
Sprigg, R.C. 1988. On the 1946 discovery of the Precambrian Ediacaran fossil fauna in South Australia. Earth Sciences History 7:46–51.
Zhuravlev, A.Y. 1993. Were Ediacaran Vendobionta multicellulars? Neues Jahrbuch für Geologie und Paläontologie 190:299–314.
ADDITIONAL WEB RESOURCES
For a treasure trove of information on Aspidella, see:
http://fossilslanark.blogspot.com/2019/05/photographs-of-gsc-221-one-of-alexander.html
And for a nice write-up on the Charnia saga, see:
https://www.damninteresting.com/chronicles-of-charnia/